Legal basis of herbal medicine regulation
All medicinal products including herbal products must have a registration or marketing authorization in accordance with EU Directive 2001/83/EC and amending directives Directive 2004/24/EC and Directive 2004/27/EC before they can be marketed in the EU/EEA states.
- Directive 2001/83/EC of the European Parliament and of the Council of 6 November 2001 on the Community code relating to medicinal products for human use states:
“No medicinal product may be placed on the market of a Member State unless a marketing authorization has been issued by the competent authorities of that Member State in accordance with this Directive or an authorization has been granted in accordance with Regulation (EEC) No 2309/93.”
- Directive 2004/24/EC (The Directive on Traditional Herbal Medicinal Products/ the Herbal Directive), of the European Parliament and of the Council, of 31 March 2004 amending, as regards traditional herbal medicinal products, Directive 2001/83/EC on the Community code relating to medicinal products for human use introduces a simplified registration procedure for traditional herbal medicinal products:
The simplified procedure clarifies differences and uncertainties in the status of traditional herbal medicinal products and facilitates the free movement of these products through harmonised rules.
The Herbal Directive came into full effect in 2011 and replaces most existing member state regulations. It creates a unified licensing system for traditional herbal medicine products.
The registration procedure is intended for herbal medicinal products with a long tradition of medicinal use (at least 30 years, of which 15 must usually have been in the EU), which do not fulfil the "well established use" requirements for marketing authorisation, i.e. published scientific literature on recognised efficacy and safety.
The Herbal Directive applies to manufactured herbal medicinal products sold over the counter, prohibiting the continued sale of unlicensed products. This means that an individual state within the EU/ EEA area is not free to uphold deviating national regulation of (herbal) medicinal products that are in violation of the relevant EU Directives.
In the eight countries which are not member states of the EU/ EFTA, the regulation of herbal medicinal products follows the EU directive/amendment procedures with small variations (Albania, Bosnia and Herzegovina, Croatia, Montenegro, North Macedonia and Serbia), partly (Turkey) or follows national regulation (Israel).
Please note that EU regulations of herbal medicinal products also applies to anthroposophic, traditional Chinese and Ayurvedic herbal medicinal products but not to food (dietary) supplements. EU regulation of food supplements can be found in Directive 2002/46/EC of the European Parliament and of the Council of 10 June 2002 on the approximation of the laws of the Member States relating to food supplements. For homeopathic products please refer to this page.
Institutions relevant for herbal medicine regulation
The European Medicines Agency (EMA) is responsible for the scientific evaluation of medicines in the European Union. Specifically, the EMA is responsible for the centralized procedure and for arbitration in cases where there is a disagreement between Member States in the ‘mutual-recognition’ and ‘decentralized’ procedures.
Opinions and decisions made by the EMA are transmitted to the European Commission for final approval. As the EU’s executive body, the European Commission has the ultimate authority for granting marketing authorizations in the EU.
The Committee on Herbal Medicinal Products (HMPC) is one of six Scientific Committees of the EMA. The HMPC compiles and assesses scientific data on herbal substances, preparations and combinations with a focus on safety and efficacy (European Union monographs).
A European Union (EU) herbal monograph (formerly known as Community herbal monograph) contains the HMPC's scientific opinion on safety and efficacy data about a herbal substance and its preparations intended for medicinal use. The HMPC evaluates all available information, including non-clinical and clinical data, but also documented long-standing use and experience in the EU.
For regulatory pathways of herbal medicinal products please see EMA’s website on herbal medicinal products.
Supervision of herbal medicine practice
Delivery of healthcare and therefore the regulation of clinical practice and practitioners is the responsibility of the respective member states. There is currently no existing statutory regulation system for herbal practitioners.
Herbal medicine/ Phytotherapy as a treatment is directly regulated in 10 of 39 countries:
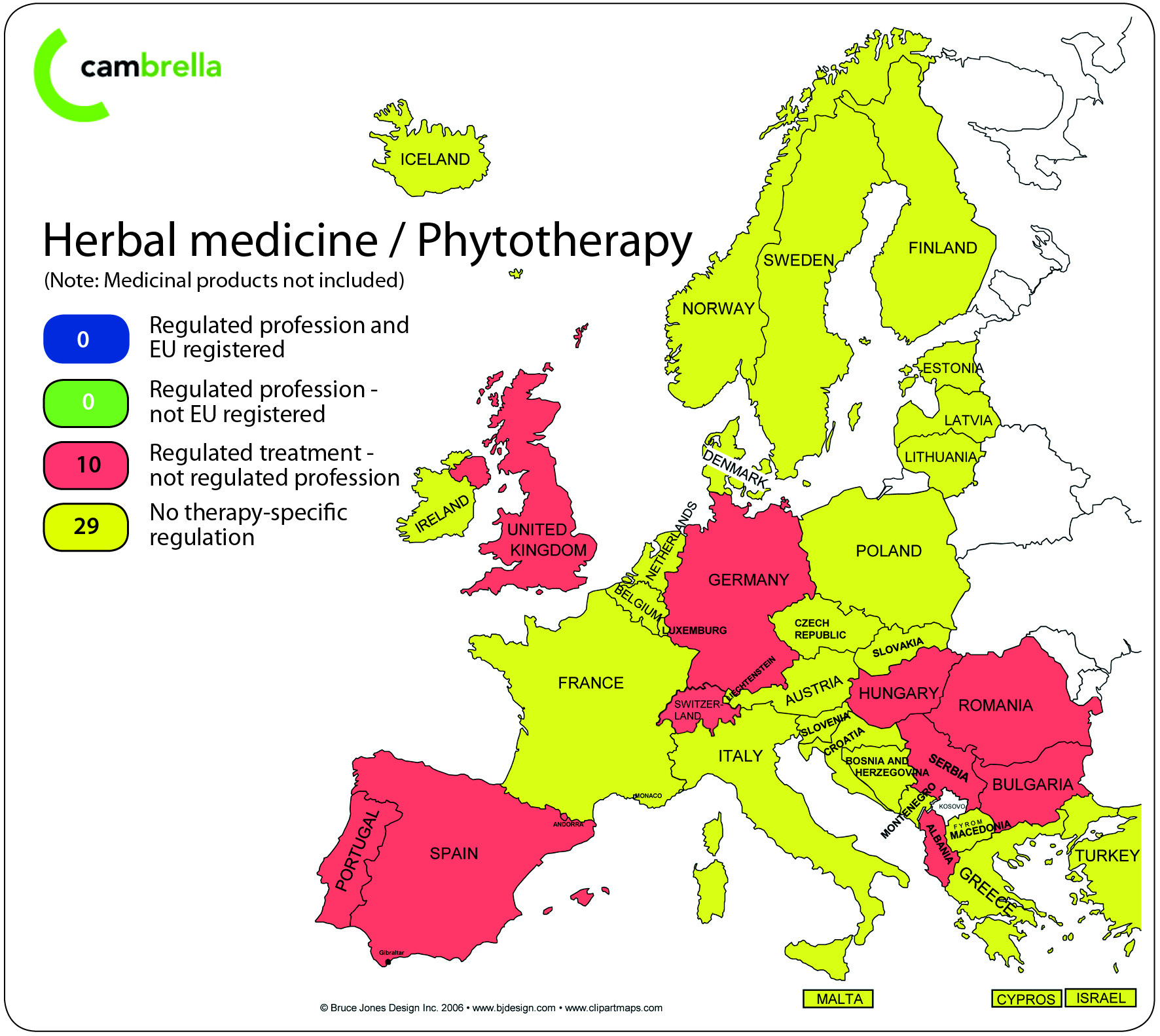
-
In Albania the law on health care regulates herbal medicine as an alternative therapeutic system
-
In Bulgaria the health law includes educational regulations for herbal medicine providers.
-
In Germany the treatment is common among medical doctors and Heilpraktikers; it is regulated under the Medicines Act as a distinct therapeutic system (“Besondere Therapierichtung”.
-
In Hungary the CAM law regulates phytotherapy.
-
In Portugal phytotherapy is regulated by law on the provision of non-conventional therapies.
-
In Romania herbal practice is recognized as a CAM therapy under the CAM law.
-
In Serbia the CAM by law lists phytotherapy as “a method of treatment suitable for practice”.
-
In Spain a new act is in progress, and only medical doctors are allowed to practise “nature medicine as a medical act”
-
In Switzerland herbal medicine is included in federal laws and detailed regulation is delegated to medical and non-medical associations.
-
In UK regulation of herbal medicine practitioners is voluntary. Statutory regulation is in progress
Reimbursement
Reimbursement of herbal medicines is subject to national healthcare policy. It is largely dependent on the individual contracts with the respective health insurers.
Go directly to the regulation of herbal medicine/ phytotherapy in a specific country:
Albania - Austria – Belgium – Bosnia and Herzegovina – Bulgaria – Croatia – Cyprus – Czech Republic – Denmark – Estonia – Finland – France - Germany – Greece– Hungary – Iceland – Ireland – Israel - Italy - Latvia – Liechtenstein – Lithuania – Luxembourg – Macedonia – Malta – Montenegro – Netherlands – Norway – Poland – Portugal – Romania – Serbia – Slovakia – Slovenia – Spain – Sweden – Switzerland – Turkey – United Kingdom
CAMbrella - A pan‐European research network for Complementary and Alternative Medicine. Regulation of herbal and homeopathic medicinal products. Deliverable 9 – Report No.2, December 31, 2012.
European Medicines Agency (EMA)
Herbal Medicinal Products Committee (HMPC)